by Chris W | Sep 3, 2019 | Medical
Yesterday, the NZ National Heart Foundation published a web article titled ‘Biggest NZ nationwide study has global impact’.
It outlined the findings of a two-year-long, national, randomised trial to examine the effect of providing ambulatory oxygen to patients suspected of suffering a heart attack. This has long been a practice observed due to its suspected benefit, however this benefit has never been confirmed.
This study is the largest randomised trial to date in NZ, involving 40,000 patients, and it was made possible by leveraging off of the ANZACS-QI system, a national cardiology registry provided by Enigma to the NZ Ministry of Health, and deployed into each NZ hospital, nationwide.
The core ANZACS-QI registry system has been extended, using funding from the ANZACS-QI Registry Trials Group to include a ‘studies module’ which provides bolt-on templates for a range of associated studies.
The studies module allows patients from the core ANZACS-QI cohort to be included in appropriate research cohorts, and for additional, study-specific information to be collected on them, perhaps including study-specific workflows and additional subsequent (post-discharge) data capture. It also provides the potential for external healthcare providers to contribute additional information on patients after they have left the hospital setting.
For this study, data relating to Oxygen-as-a-therapy was retrospectively collected on all patients who were entered into the ANZACS-QI Acute Coronary Syndrome (ACS) data collections, on a single randomised day each month. Oxygen fields were present on all days, for all patients, but were made mandatory for patients who entered the hospital system on those specific, randomly selected days. In this way, the normal clinical practice of providers was not affected nor influenced in any way, by the presence of the study.
For more details on the study’s findings visit: https://www.heartfoundation.org.nz/about-us/news/media-releases/biggest-nz-nationwide-study-has-global-impact
Enigma is proud to have played a part in this and wishes to acknowledge and thank the NHF for their support of this study.
by Chris W | Nov 25, 2016 | Medical
Enigma is delighted to have enabled TestSafe access for the Auckland and Northern Region’s ANZACS-QI users. Historically this access has been available only for CMDHB’s ‘Acute Predict’ users and dates back to around 2010 – (pre-TestSafe days). Over the past few years there have been several efforts to work through the permissions required to ‘flick the switch’ for the other DHB’s within the TestSafe catchment area.
Fair to say that the wheels turn slowly… Over four years later, we received the formal go-ahead which we required, and last week ‘flicked the switch’ to provide this same service to the others in the region. This represents a rather large milestone for us, and as such we’re delighted.
What this means for the end users – it’s simple really; they don’t need to transpose results from one screen, into another window. The systems are now ‘connected’ and at the click of a button the ANZACS-QI system is able to perform a back-end lookup into TestSafe, and pull the relevant labs directly into the ANZACS-QI Registry’s forms.
It saves valuable time, reduces effort and removes transposition errors. It will help to capture the highest possible quality Registry data.
We’d like to thank the staff at health Alliance who helped significantly with this most recent effort to work through the required processes. In particular we need to recognise Adele Nairn (health Alliance), without whom we would not have achieved this. We’d also like to thank Sarah Thirlwall (CMH), Leanne Elder (CMH), Barbara O’Shaughnessy (WDHB) and Stuart Bloomfield (WDHB) for the various parts which they played in sponsoring work, organising assistance and completing the PIA work too.
For more information on ANZACS-QI, feel free to click here: http://www.enigma.co.nz/predict-medical/anzacs-qi/
by Chris W | Feb 29, 2016 | Medical
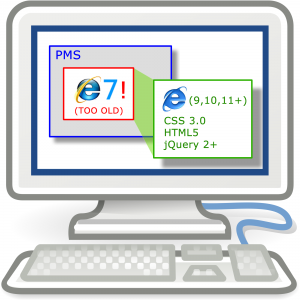
So, in recent years, there have been many advancements in browser standards and technologies which they support; two major ones are CSS 3.0 and the use of HTML5. Neither of these technologies are supported by older browsers such as MSIE7 and MSIE8.
Both MSIE7 and MSIE8 have been defunct for some time now, and are well past their ‘end of life’ with Microsoft. This means that they no longer provide updates, security patches or support for these old browsers. There is no way that anyone in any business or professional setting ought to still be running these browsers if they are capable of connecting to an internet based source of content. (Legacy APP support on an isolated network might be the only excusable reason to want to run these versions).
In some settings, a ‘WebBrowser control’ is used within other applications to allow the desktop based application to access, and view web based content. In particular, Microsoft provided such a control, and allow MSIE to be invoked in an embedded manner within other applications. This is the approach used by both Medtech and MyPractice.
The ‘WebBrowser control’ ships with some standard, default settings which force certain behaviours unless altered. The good news is that on a per-application basis, registry entries can be added to the machine which uses these controls, to nominate what emulated version the current desktop browser ought to be run as. This is where things get a little confusing and certainly more detailed…
If you’re running Windows 7, Windows 8 or Windows 10, the chances of you running MSIE8 as your desktop browser are almost nil. However, the WebBrowser control’s default settings force even these newer browsers to run in an emulation mode, pretending, and limiting the browser’s function to that of MSIE7. Clearly this is a hampering default setting and one which severely limits the usefulness of the embedded browser.
This is a major issue for software development companies who maintain modern approaches to development and deployment. Enigma, as one of those companies is focussed on delivering the best in terms of usability and end-user-experience as well as security. This means that we need to be able to use the most modern application development approaches and frameworks. – Simply put, we cannot while your embedded browsers are being forced to continue to run in this MSIE7 compatibility mode.
So, we’re forced to find a way to allow you to use your native browser, the one installed on your desktop, your current version. – A linked popup window appears to be an interim solution. It’s not the most appropriate answer, but it will allow us to continue with delivering decent software, in an integrated manner. We’ll only have to take this approach until the PMS vendors have figured out a suitable way to allow all form vendors to make use of the latest versions within the PMS.
Some FAQs:
— But, can’t that happen now? You mentioned Registry settings?…
Yes, it can. By adding a changed registry setting, your PMS vendor can opt into allowing the very latest browser which you have installed on your desktop to be used within the PMS. This is something which they are capable of delivering out to you within a version update.
— So, why have they not done this?…
There may be more than one single reason, but the easiest to explain is that there is concern over whether all forms which have been previously deployed will continue to work in this latest version. So until they have managed to talk to all form vendors to assess their readiness, they appear reluctant to change these settings.
— Is anything being done about this?
Yes, there have been a series of discussions which date back over 18 months on this topic. Also, we have started to make plans to use this popup method of window-linking between the window within the PMS, and the popup window. Using this approach, we’re able to talk back to the PMS, to perform our approved methods of integration with the PMS, using data, and writing back etc; while making the most of the newer capabilities and functions of your more modern browser.
— So this is a temporary thing?
Yes, we very much hope so. – There are some potential issues with this approach which make it a little more clumsy for end-users, you might lose sight of where your window is etc; also if you close the patient within the PMS, there is nothing we can do apart from simply close the browser content, we will attempt to warn you that you have a patient based window still open before you close it, but if you do close it, and if you have already entered content into the popup window, then you will lose that work, sorry. Arguably, this is no worse than the current workflow, if you close a patient with an open embedded window, and accept it can be closed without first saving / submitting that work, you could lose that too.
— How long until this is sorted properly?
We cannot give you an idea of timeframes as the work is outside of our control.
— Which PMS versions is this currently a problem in?
At the time of writing: We have experienced MSIE7 version mode being forced in Medtech32, MSIE8 mode is being forced within Evolution. – Neither MSIE7 or MSIE8 is new enough to support our current needs, we will use popup windows in all PMS systems where their embedded browser versions are not new enough to support current development approaches. MyPractice does not apply any version-mode limitations.
If you need further information on this topic, please contact the team @ Enigma: [email protected]
by Chris W | Feb 12, 2016 | General, Medical

ANZACS-QI is a National system delivered within NZ to all of the public and private Coronary Care units and Cathlabs. It forms a National Registry to collect data on all patients who present with suspected Acute Coronary Syndrome, as well as those who are treated within Cathlabs, and who receive device implants as part of their cardiac-care treatment. The system is based on the PREDICT platform and was developed by Enigma in conjunction with Dr Andrew Kerr with funding from CMDHB. It was further developed later, with the NZ Cardiac Network, under contract with New Zealand’s Ministry of Health and has been delivered nationally in partnership with NIHI.
The following excerpts are taken from an editorial article [Mark Webster; ‘Early angiography and revascularisation for acute coronary syndromes in New Zealand’, NZMJ – 8th Jan, 2016] – Full article is available here with subscription: https://www.nzma.org.nz/journal/read-the-journal/all-issues/2010-2019/2016/vol-129-no-1428-8-january-2016/6678
Excerpts taken from the referenced editorial article:
ANZACS-QI is a rigorous, prospective and comprehensive database on all patients with ACS undergoing angiography. … The 3-day target time for angiography in ACS patients was an excellent choice as a KPI: clinically relevant, achievable and with the potential to save money. This has proven to be the case.
ANZACS-QI is now providing a wealth of information about the management of coronary disease in New Zealand. Merging data from other sources, particularly the national mortality, hospital discharge diagnosis codes and pharmacy registries, creates a powerful tool for predicting longer-term outcomes. …
…
The potential for ANZACS-QI extends well beyond short-term audit and quality control of local practice. Research can be under-taken by adding database fields for the duration of a study, and national registries used to collect relevant endpoints. … New Zealand is now well placed for such research; being small and somewhat isolated is, for once, to our advantage. Beyond the usual randomised trials with individual patient consent, there is also the potential to undertake systems research, comparing ‘routine’ practices applied to large groups of patients.
In summary, ANZACS-QI is an example of money well spent in a public health service looking to achieve both optimal clinical outcomes and efficient health service delivery. Without good data, it is easy to repeat the mistakes of the past. As Lord Kelvin said “to measure is to know”. It is vital that we evaluate our practice in an ongoing and rigorous manner … ; it is also important to maintain a healthy scepticism regarding our currently-held beliefs.
Further information on the ANZACS-QI system is available here: http://www.enigma.co.nz/predict-medical/anzacs-qi/
Further information on the extensions of this base system to service research / study requirements is available here: http://www.enigma.co.nz/predict-medical/anzacs-qi-registry-trials/
About Mark Webster: Mark works as a Consultant Cardiologist, Director, Cardiac Catheterisation Laboratories Auckland City Hospital and is Hon. Associate Professor of Medicine, University of Auckland.
by Chris W | Dec 23, 2015 | General, Medical


PREDICT (Enigma) / VIEW (University of Auckland)
Partnership statement; looking forward to 2016.
23rd Dec, 2015
The University of Auckland’s, School of Population Health and Enigma Solutions Ltd, have been in partnership with PREDICT, under the ‘VIEW’ programme of work and its predecessors, since 2002. This partnership continues to support continued research efforts, funded by various, multi-year HRC grants and is of NZ National interest.
This valuable, back-end, research based work sets PREDICT apart from other tools available for clinical use in New Zealand; there is no comparable tool having research based linkages as a primary goal.
Since its formation the aim of the partnership has been to deliver robust, evidence based guidance (mainly related to cardiovascular disease and diabetes) into the patient’s care setting to:
- aid consistency and appropriateness of delivered care (minimise variations leading to under or over-treatment);
- collect data relating to service delivery and through linkage to national (patient outcome) datasets, and thereby
- validate current guideline recommendations and fit for the NZ population and
- improve the specificity of risk prediction approaches for the NZ population by creating a NZ specific set of algorithms based on observed incidence rates.
Secondary aims have included:
- the creation of secondary risk prediction equations for those who have already had a CVD event;
- to map variations in treatment and risk profiles geographically for NZ
Organisations that use Predict are invited to opt-in to participate within the research programmes by contributing the data which is collected during their routine clinical services. This data is handled appropriately with sophisticated anonymisation routines and complies with the National Ethics Committee approval granted to the programme.
There are now over 500,000 de-identified individual New Zealand patient profiles within the PREDICT cohort that are available to the VIEW programme for their analysis and linkage to NZ Ministry of Health provided ‘outcomes data’. -(vs Framingham’s original cohort of just 5,209 patients.)
VIEW has worked towards generating a NZ specific, validated risk prediction algorithm which can be more accurately applied to our own specific population and is more relevant to today’s other, wider risk factors such as social determinants of health and medication management. Currently, at the time of writing, an alternative, NZ specific, general population, primary prevention algorithm has been independently peer reviewed in a process organised by the Heart Foundation and it is expected that it will be available in the new year (2016).
Early testing and implementation for demonstration purposes of these new algorithms has begun alongside the peer review process with some interesting findings.
Additionally secondary prevention equations (risk assessments for those who have already had a CVD event) have also been independently peer-reviewed and will be available in 2016 following completion of testing processes.
A third set of new, validated equations are expected to be available specifically for Maori and for Diabetic patients in 2016. This work is expected to be on-going with refinements made to risk prediction approaches, creating more personalised, tailored outputs for NZ’s sub- populations.
In 2016, Enigma and VIEW will be launching Your Heart Engine (YHE), a combined set of risk assessment and risk visualisation tools for CVD. This is a centralised service into which each of these newly developed risk calculators will be released. Coupled with this, the Your Heart Forecast tool will be modified to support new scenarios, such as the secondary prevention equations.
CVD / Diabetes electronic clinical decision support (eCDS) tools use an individual’s calculated risk as one of the management determinants. The YHE approach is capable of supplying the risk profiles into the eCDS tools so that their management rules can provide management advice according to a patient’s estimated risk score.. PREDICT CVDDM will be modified to use the YHE content in this manner, workplace instances of PREDICT already run in this way.
VIEW and Enigma remain fully committed to the continued support and maintenance of the PREDICT eCDS management components; consistent and appropriate (evidence based) management of patient’s risk remains the key aim of the programme. The development of Your Heart Engine is one step towards making these management tools simpler, quicker, more cost effective and more consistent; it also strengthens our current focus on quality assurance, doing the job well and doing it once.
The Your Heart Engine (which will include relevant access to the graphic risk communication tools) will be commercially available as a service for other eCDS / CVD RA front-end tools to use. Linking into this centralised service will provide a consistent risk assessment approach across multiple tools and across New Zealand. Enigma and VIEW have an excellent reputation for high quality, clinically orientated implementation of these tools and it is exciting to be able to offer these services out to a wider set of users. For more information visit: https://secure.YourHeartEngine.co.nz

Through YHE, other tools, vendors and groups of end users will have an option to opt-in to these VIEW based research programmes, this is something which will be fully explored with each new group who adopt YHE as a service.
By continuing to support PREDICT products, you are helping to contribute to these research efforts, which in turn is now paying dividends for NZ’s evidence based, population health approaches.
2016 should be an exciting year, we’re grateful for your support to-date and hope to continue to deliver quality products which help to support your clinical service delivery for your patients.
Merry Christmas and Happy New Year from your friends at Enigma and our UoA VIEW team collaborators.
For a PDF version of this document: click here.
by Chris W | Nov 11, 2015 | Medical
Enigma will be attending and exhibiting at the IHIC conference in Melbourne, 17th & 18th November, 2015. We will be a proud part of the New Zealand contingent on the NZTE stand showcasing 7 NZ vendors to the Australian market.
As part of our attendance there, we will be running a prize draw / give away of an iPad Mini 2, 32GB model.

The winner will be announced on this page after the draw has taken place.
Good luck, and we hope to see as many of you as possible at the conference!





